NATIVA PRENATALE
The Test of non-invasive prenatal screening capable of identifying the main chromosomal abnormalities present in the fetus and the gender of the unborn child
NATIVA is the non-invasive prenatal test clinically validated on thousands of pregnancies, it provides precise and punctual information on the aneuploidies of all chromosomes, chromosome deletions and duplications of size ≥ 7 Mb and six microdeletions.
NATIVA, thanks to the greater sensitivity and specificity compared to conventional screening tests, decreases the use of invasive diagnostic tests, reducing the risk of miscarriage related to them.
Si esegue con un prelievo di sangue materno, attraverso il quale è possibile analizzare i frammenti del DNA fetale circolante.
NATIVA può essere effettuato a partire dalla 10a settimana di gestazione e, a differenza dei test diagnostici invasivi, non presenta rischi per la mamma e per il bambino.
To book NATIVA PRENATALE send a mail to customerservice@xbiogem.it
What does it analyze

Analysed at the BioRep laboratories, in Italy

Analysed at the BioRep laboratories, in Italy

Analysed at the BioRep laboratories, in Italy

Analysed at the BioRep laboratories, in Italy
How does it work
F.A.Q. - NATIVA PRENATAL
The test is indicated in case of:
It can also be performed in case of:
The NATIVA test is not a substitute for invasive prenatal diagnosis, such as amniocentesis and CVS, but it is a screening test whose results must be evaluated in the context of the clinical picture and anamnestic of the pregnant woman.
The test does not detect the state of health of the mother and is not able to highlight the alterations of the chromosomes not analyzed, the balanced and unbalanced structural chromosomal rearrangements, the polyploidies.
The test should be included in a pregnancy path agreed with your doctor.
The case of twin pregnancies:
The test is NOT recommended in case of:
The NATIVA test is performed on a simple maternal blood sample, it is non-invasive and does not involve any risk for the pregnant woman and the child. NATIVA analyzes the fragments of fetal DNA that circulate freely in maternal blood. The procedure is simple and the result is obtained in 5/12 working days from receipt of the sample at the laboratory.
For the NATIVA and NATIVA NEXT tests it takes 5 working days from when the sample arrives in the laboratory, while for the NATIVA PLUS and NATIVA KARYON tests it takes 10-12 working days from when the sample reaches the laboratory.
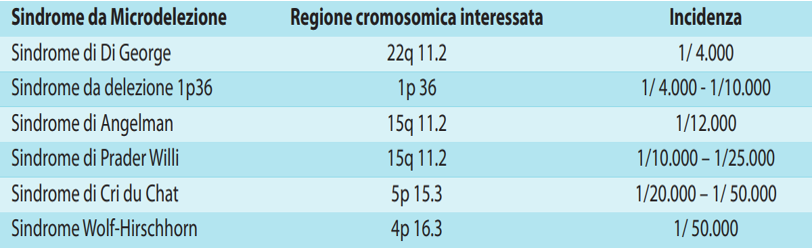
NATIVA PLUS and NATIVA KARYON analyze the presence of microdeletions, structural anomalies of the chromosomes caused by the loss of small portions of DNA. Some microdeletions are specific to well-characterized chromosomal regions and are associated with particular syndromes. Microdeletion syndromes are clinically recognizable pathologies, characterized by a peculiar phenotype, and include the syndromes listed in the table.
Rare Autosomal Aneuploidies (RAAs) are numerical alterations (presence of one chromosome more or less than the normal karyotype human) that can occur in all chromosomes. Partial duplications and deletions (CNVs) are chromosomal abnormalities that have more or less DNA portions than the normal karyotype and can occur in any chromosomal region.
NATIVA NEXT analyzes all RAAs and CNVs >7Mb in size
The incidence of RAAs in early pregnancy is estimated at 0.34%. The incidence of CNVs >7Mb is estimated at 0.10% which together with RAAs (0.34%) make up 0.44% of the incidences of total chromosomal conditions. Compared to 0.50% of the estimated incidence of the most common trisomies 21, 18, 13.
It is possible to request the reimbursement in the form of indirect compensation, however it is advisable to first check the reimbursement of the service with your health insurance company.
You can contact XBIOGem on +39 06 3015 8224 Monday to Thursday from 8:30 to 16:00 and Friday from 8:30 to 14:00.
