XCLARIS FPG500
Tests for the genomic profiling of tumors in order to offer access to the best treatments currently available
Why is it useful to do XCLARIS FPG500?
XCLARIS FPG 500 is a test that allows you to perform a broad genomic profiling in patients diagnosed with cancer, obtaining:
To book FPG500 send an email to customerservice@xbiogem.it
What does it analyze

It identifies genomic alterations present at both the DNA and RNA levels. In particular, XCLARIS FPG 500 at the DNA level identifies single nucleotide variants (SNVs), insertions/deletions (indels) and copy number variations (CNVs) present in 523 genes; while at the RNA level it is able to identify both known and unknown fusions, as well as splicing variants, in 55 genes.

It identifies genomic alterations present at both the DNA and RNA levels. In particular, XCLARIS FPG 50 at the DNA level identifies single nucleotide "hot spots" variants (SNVs), insertions/deletions (indels) and copy number variations (CNVs) in 35 genes; while at the RNA level it is able to identify both known and unknown fusions, as well as detect cancer-associated isoforms and alternative splicing events in 17 genes.
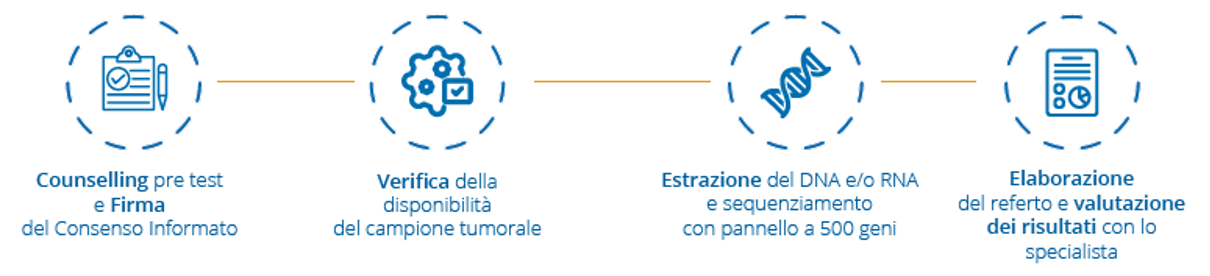
How does it work
F.A.Q. XCLARIS FPG500
Genomic profiling is currently recommended in numerous neoplasms including: lung, ovary, prostate, pancreas, melanoma, breast, GIST (gastro intestinal stromal tumors), colon, thyroid.
Before carrying out the test, it is particularly advisable to undergo a consultation with a specialist, to discuss the purpose and the implications of the possible results that will derive from the execution of the test.
The test can be performed both on DNA/RNA extracted from tumor tissue (tumor specimens fixed in formalin fixed and paraffin-embedded (FFPE), core needle biopsies, fine needle aspiration and cytological tissue) and on circulating DNA (ctDNA ) extracted from plasma. Depending on the type, the sample must be collected according to the methods indicated on the sheet included in the package.
N.B. ** The sample of the histological preparation on which to carry out the analysis, if the patient is external to the Fondazione Policlinico Universitario A. Gemelli IRCCS, must be accompanied by a histological report from the external center indicating the percentage of tumor cells present. If the center does not provide the report, the patient must carry out a re-reading of the exam at the Fondazione Policlinico Universitario A. Gemelli IRCCS, at an additional cost ** .
The method of analysis is the Next Generation Sequencing (NGS) technique, which allows to sequence a high number of genes in a short time. It is performed at the laboratories of the Genomics Research Core Facility of Fondazione Policlinico Universitario Agostino Gemelli IRCCS.
The sample, after carrying out the analysis, will be destroyed according to current legislation. All data and related results are managed in full guarantee of confidentiality.
For the execution of the tests, 20 working days are foreseen from when the biological sample arrives at the laboratory.
For a correct interpretation of the results, it is advisable to seek advice from a specialist doctor.
For XCLARIS FPG500 the cost is €1500, for XCLARIS FPG50 the cost is €1250.
An additional cost of €200 will be requested if the tumor sample provided does not meet the requirements specified in the sheet included in the test package.
It is possible to request the reimbursement in the form of indirect compensation, however it is advisable to first check the reimbursement of the service with your health insurance company.
You can contact XBIOGem on +39 06 3015 8224 from Monday to Thursday from 8.30 to 16:00 and on Friday from 8:30 to 14:00.
