XCLARIS Onco Screening Test
XCLARIS Onco Screening Test is a genetic test aimed at the search for molecular alterations of a large panel of genes, in particular BRCA1 and BRCA2, which are associated with a greater risk of developing some cancers.
Why is it useful to do XCLARIS Onco Screening Test?
XCLARIS Onco Screening Test allows you to identify people who have an increased risk of developing cancer of the ovary , breast, uterus, pancreas, prostate and Lynch Syndrome at aiming to direct them towards prevention primary and secondary programs.
XCLARIS also has therapeutic implications for subjects suffering from certain cancers as it could predict the effectiveness of some treatments and identifies molecular targets for a personalized therapeutic treatment .
XCLARIS Onco Screening Test can be performed on a sample of peripheral blood, saliva or tissue which is analyzed at the Molecular Diagnostics and Chemical Genomics, Biochemistry and Molecular Biology Laboratories of Policlinico Universitario Agostino Gemelli IRCCS.
For the reporting of the Test there are 15 to 30 working days from when the biological sample arrives in the laboratory.
To book XCLARIS send an e-mail to customerservice@xbiogem.it
What does it analyze

Analysis of BRCA1/2 genes
The test can be performed on blood, on tumor tissue or on sample BRCA1/2 salivary genes

Targeted search for pathogenic variant in the BRCA1/2 genes
Test that can be performed on blood, tumor tissue or salivary sample
Analysis of structural rearrangements in BRCA1/2 genes by MLPA technique
Test that can be performed on blood and tumor tissue

Analysis of 12 predisposing genes for BRCA 1/2 negative ovarian and / or breast tumors: ATM, BARD1, CDH1, PALB2, PTEN, RAD51C, RAD51D, STK11, TP53, CHEK2, NBN, BRIP
Analyzes 16 genes most involved in the homologous recombination pathway predisposing for ovarian, breast and prostate cancers: ATM, BARD1, BRCA1, BRCA2, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L, TP53
Analysis of 26 genes associated with Lynch syndrome and tumors of the ovary, breast, uterus and pancreas: ABRAXASI, ATM, APC, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MRE11, MSH2 , MSH6, MUTYH, NBN, PALB2, PIK3CA, PMS2 - PMS2CL, PTEN, RAD50, RAD51C, RAD51D, STK11, TP53, XRCC2
All solutions can be performed on both blood and tissue
How does it work
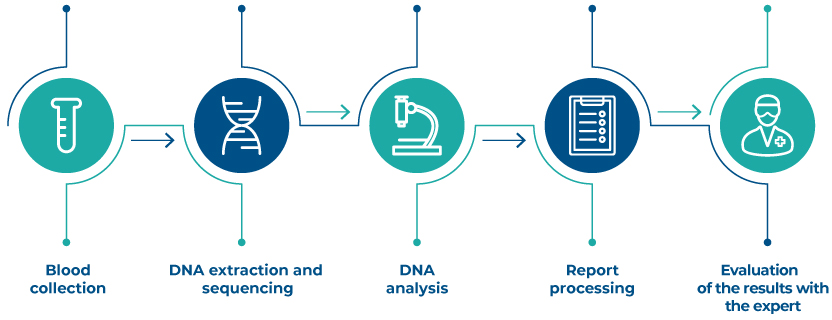
F.A.Q. XCLARIS Onco Screening Test
XCLARIS Onco Screening BRCA PANEL is particularly indicated in:
- -Familiarity for breast, ovarian, pancreatic or prostate cancer in three or more cases in the same family branch;
- -patients with non-mucinous and non-borderline ovarian carcinoma, fallopian tube carcinoma or primary peritoneal carcinoma;
- -Woman with breast cancer before the age of 36;
- -Woman with breast cancer and ovarian cancer;
- -Woman with triple negative breast cancer under the age of 60;
- -Woman with bilateral breast cancer less than 50 years old;
- -Male breast cancer;
- -Patients with metastatic pancreatic cancer;
XCLARIS Onco Screening Test uses first and second generation sequencing technologies. The technology used for carrying out the test is CE-IVD certified.
XCLARIS Onco Screening Test can give a "Positive" outcome, that is to detect one or more variants of clear pathogenetic significance of the investigated genes, or a "Negative" outcome, that is, identify only alterations of benign clinical significance or be "Non Informative" in the case which highlights variants of uncertain and / or unknown clinical significance.
Furthermore, the finding of a pathogenetic variant in patients with BRCA1 / 2 ovarian, breast, pancreatic and prostate cancer also has therapeutic implications as it could predict the effectiveness of some chemotherapy treatments and PARP inhibitors.
The sample, after carrying out the analysis, will be destroyed in accordance with current legislation. All data and related results are managed with full guarantee of confidentiality.
To access XCLARIS it is necessary to carry out an informative consultation before and after the test with a specialist doctor (geneticist, oncologist or gynecologist oncologist), the test can be purchased:
- - Online on the website of XBIOGem;
- - By e-mail to customerservice@xbiogem.it ;
- - By calling +39 06 3015 8224 active from Monday to Thursday from 8:30 a.m. to 4:00 p.m. and on Friday from 8:30 a.m. to 2:00 p.m.;
- - Directly on the website at www.xclaris.com;
- - Through the booking number of the private practice of the Policlinico Gemelli at +39 06 8881 8881 active from Monday to Friday from 8:30 a.m. to 6:00 p.m. and on Saturday from 9:00 a.m. to 1:00 p.m.
The price varies according to the type of test performed:
- XCLARIS BRCA PANEL (BRCA 1 e 2)
- - Germ Test - Test on Blood: € 390
- - Germ Test - Saliva Test: € 390
- - Somatic Test - Test on Tissue: € 400
- XCLARIS BRCA TARGETED
- - Germ Test- Test on Blood: € 280
- - Germ Test - Saliva Test: € 280
- XCLARIS BRCA MULTI PANEL
- - 12 genes (Germ Test- Test on Blood): € 510
- - 12 genes (Somatic Test- Test on Tissue): € 590
- - 16 genes (Germ Test- Test on Blood): € 670
- - 16 genes (Somatic Test- Test on Tissue): € 830
- - 26 genes (Germ Test- Test on Blood): € 780
- - 26 genes (Somatic Test- Test on Tissue): € 930
The cost includes the execution of the test and a pre and post test video consultation performed by a specialist doctor (geneticist, oncologist or gynecologist oncologist) of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS.
It is possible to request reimbursement in the form of indirect compensation, however it is advisable to first check with your health insurance the reimbursement of the service
It is possible to contact XBIOGem at +39 06 3015 8224 from Monday to Thursday from 8.30 a.m. to 4:00 p.m. and on Friday from 8:30 a.m. to 2:00 p.m.
